Plastic waste represents one of the most pressing environmental and resource challenges of our time. While recycling efforts are crucial, conventional mechanical methods have limitations, especially for mixed, contaminated, or degraded plastics. This is where chemical recycling, specifically catalytic pyrolysis, emerges as a transformative solution. Moving beyond simple heating, it introduces a molecular architect—the catalyst—to precisely deconstruct plastic polymers. Let’s delve into the fascinating molecular-level art of breaking carbon chains, a process that makes advanced plastic pyrolysis plant operations not just possible, but efficient and targeted.
1. The Foundation: Thermal Pyrolysis vs. Catalytic Pyrolysis
First, a baseline. In standard thermalpyrolysis, plastics are heated to high temperatures (typically 400-600°C) in an oxygen-free environment. Heat energy alone provides the impetus for the random scission of the long polymer chains (like polyethylene or polypropylene) into smaller fragments. This process, however, is non-selective, often yielding a very wide and complex mixture of hydrocarbons (gases, liquids, waxes). The product oil frequently requires significant upgrading for reuse.
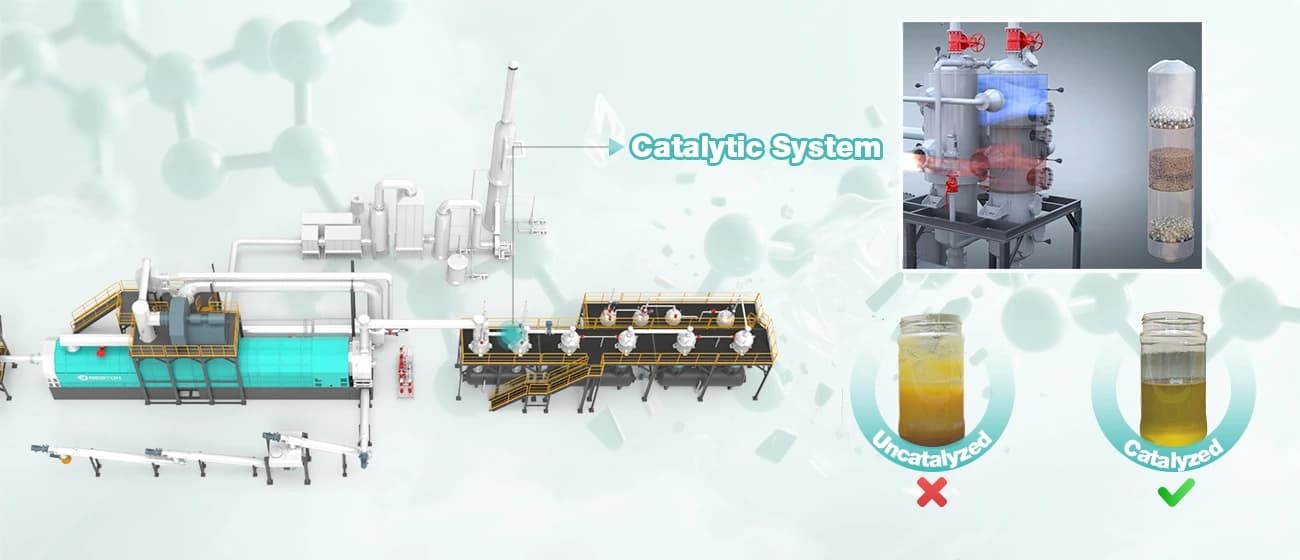
Catalyticpyrolysis revolutionizes this by introducing a catalyst—a substance that speeds up the reaction and guides its pathway without being consumed. The catalyst provides an alternative, lower-energy route for the polymer breakdown, allowing for more control, lower operational temperatures (often by 50-150°C), and crucially, a more selective and valuable product slate.
2. The Molecular Mechanism: A Step-by-Step Deconstruction
The catalyst’s work is a masterpiece of molecular interaction. Here’s how it typically unfolds at the atomic level:
- Step 1: Adsorption and Initiation. The molten plastic polymer chain comes into contact with the acidic sites (e.g., in zeolite catalysts) on the catalyst’s surface. A proton from the catalyst’s acid site interacts with the polymer, leading to the protonation of a carbon atom in the chain. This creates a positively charged, unstable intermediate (a carbocation).
- Step 2: β-Scission (The Key Break). This carbocation intermediate undergoes a rearrangement. The critical break occurs at the bond betato the positively charged carbon. This β-scission is the heart of the catalytic “art”—it’s a controlled, predictable cleavage of the carbon-carbon backbone, yielding a smaller olefin molecule (like ethylene, propylene) and a new, shorter-chain carbocation.
- Step 3: Chain Transfer and Isomerization. The newly formed carbocation can continue this cycle of β-scission, progressively “unzipping” the polymer. Simultaneously, it may undergo hydride transfer (exchanging a hydrogen with another molecule) or isomerization (rearranging its structure), which diversifies the product range. These secondary reactions are heavily influenced by the catalyst’s pore structure and acidity.
- Step 4: Desorption and Final Products. The smaller hydrocarbon molecules (now gases like propane or liquids in the gasoline/diesel range) eventually desorb from the catalyst’s active sites. The catalyst, now free, is ready for the next polymer chain. The genius lies in the catalyst’s design steering these steps toward desired products—for instance, maximizing the yield of high-value light olefins (building blocks for new plastics) or high-quality, stable liquid fuels.
3. Why It Matters: The Impact on a Modern Plastic Pyrolysis Plant
Integrating catalytic mechanisms transforms the entire system’s economics and output:
- Higher Quality Output: Catalytic pyrolysis directly produces a more deoxygenated, less waxy, and more stable liquid (higher in aromatics and iso-paraffins), closer to conventional fuel specs or chemical feedstock requirements.
- Process Efficiency: Lower required temperatures translate to significant energy savings for the plastic pyrolysis plant, reducing operating costs and the carbon footprint of the process itself.
- Targeted Product Slate: By selecting the catalyst, operators can shift the product distribution. The focus can be on maximizing chemicals (BTX, olefins) for a circular plastic economy, or on producing premium drop-in fuels.
- Challenges: Catalyst deactivation due to coking (carbon deposition) is a key challenge. This necessitates reactor designs that allow for continuous catalyst regeneration—a critical engineering consideration in any commercial-scale plastic pyrolysis plant.
Conclusion: From Random Breakdown to Precision Engineering
Catalytic plastic pyrolysis elevates waste conversion from a brute-force thermal process to a refined, molecular-level art. By orchestrating the precise scission and rearrangement of carbon chains, catalysts unlock higher value, greater efficiency, and true chemical recycling potential. The sophistication of the mechanism underscores why modern plastic pyrolysis plant technology is more than just a reactor; it’s an integrated chemical engineering system where reactor design, temperature profiles, and catalyst formulation work in concert. As catalyst science advances—towarding greater selectivity, resistance to contamination, and longevity—this elegant molecular art will be key to making plastic waste a reliable source for the circular carbon economy of the future.
Leave a Reply