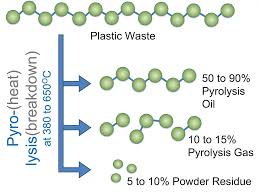
Plastic waste is a major environmental challenge, but one innovative solution is pyrolysis, a process that converts plastic into fuel, gas, or valuable chemicals. While the concept is simple—heating plastic in the absence of oxygen—the success of pyrolysis depends heavily on the process conditions. Understanding these conditions is essential to optimize efficiency, fuel quality, and environmental safety.
1. Temperature
Temperature is the most critical factor in plastic pyrolysis. Different plastics decompose at different temperatures, but generally:
- Low-temperature pyrolysis (300–400°C): Produces more liquid fuel and waxy residues.
- Medium-temperature pyrolysis (400–500°C): Balances liquid fuel and gaseous products.
- High-temperature pyrolysis (>500°C): Produces more gas and fewer liquids; often used when syngas production is the goal.
Precise temperature control ensures maximum fuel yield and prevents unwanted by-products.
2. Heating Rate
The heating rate—how quickly the plastic reaches the target temperature—affects the type and quality of the products:
- Slow heating: Produces more liquid fuel and char.
- Fast heating: Favors gas production and reduces solid residue.
Choosing the right heating rate depends on the desired output: liquid fuel or combustible gas.
3. Reaction Time
Residence time is the duration the plastic stays in the pyrolysis reactor at high temperature:
- Shorter times: Reduce char formation and favor liquid and gas products.
- Longer times: Increase solid residues but may improve cracking of larger molecules for higher-quality fuel.
Balancing time is crucial for optimizing fuel yield and reactor efficiency.
4. Pressure and Atmosphere
Pyrolysis requires an oxygen-free environment to prevent combustion. Most systems operate at:
- Atmospheric pressure: Simpler and safer for small-scale setups.
- Slightly reduced pressure or vacuum: Helps control the reaction, reduce side reactions, and improve liquid fuel yield.
Nitrogen or another inert gas is often used to create a safe, oxygen-free atmosphere.
5. Catalyst Use
Adding catalysts can enhance the plastic pyrolysis process by:
- Lowering the required reaction temperature
- Improving liquid fuel yield
- Producing fuels with better quality, such as higher calorific value or fewer impurities
Common catalysts include zeolites, alumina, or silica-based materials.
6. Type of Plastic
Different plastics behave differently under pyrolysis:
- Polyethylene (PE) & Polypropylene (PP): High liquid fuel yield, relatively easy to process.
- Polystyrene (PS): Produces high-quality liquid fuel but can foam and complicate reactor operation.
- PVC & PET: Release harmful gases like HCl or require pretreatment.
Proper sorting and pretreatment are essential for safe and efficient pyrolysis.
Conclusion
Waste plastic pyrolysis is more than just heating trash—it’s a precise chemical process where temperature, heating rate, residence time, pressure, catalysts, and plastic type all play critical roles. Optimizing these conditions ensures high-quality fuel, minimizes environmental risks, and makes the process economically viable.
As research continues, pyrolysis technology is becoming a key tool for turning plastic waste from an environmental problem into a sustainable energy resource.
Leave a Reply